T-cell engagers (TCEs) are among the most promising new modalities in cancer therapy, but limitations in efficacy and safety have been barriers to realizing their potential for patients with difficult-to-treat cancers.
We presented four posters at the American Association for Cancer Research® (AACR) Annual Meeting in April 2024 that together demonstrate how our T-cell engager platform could expand the reach of TCEs as a drug class by:
Widening the therapeutic window
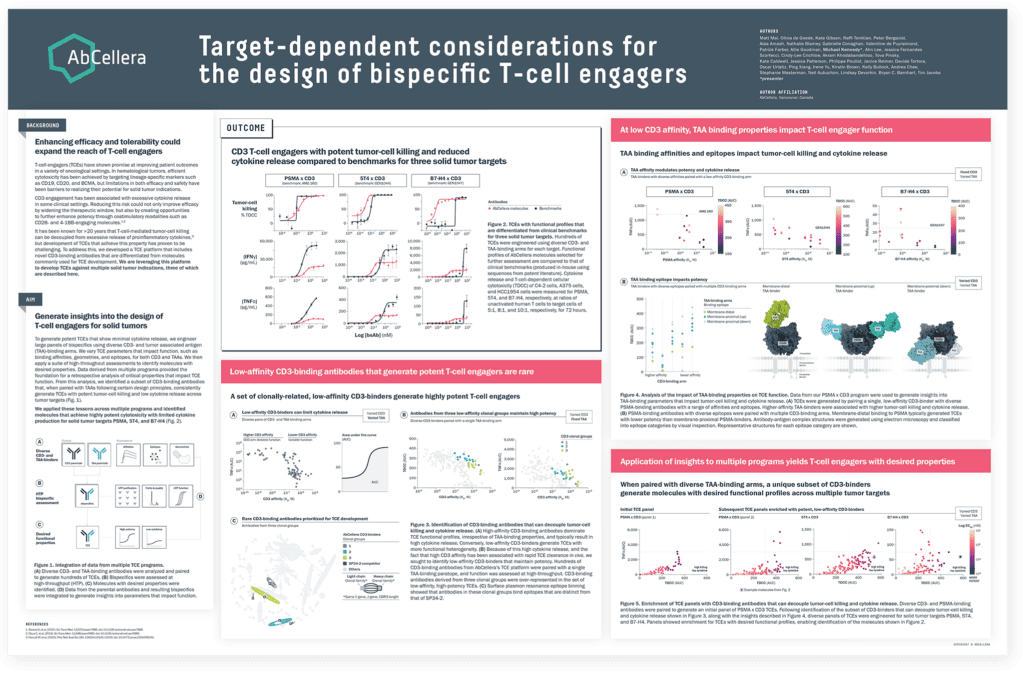
Our first poster described the generation of TCEs for three solid tumor targets, PSMA, B7-H4, and 5T4, with functional profiles that are differentiated from clinical benchmarks. These molecules were engineered using a specific set of rare CD3-binding antibodies that consistently show potent tumor-cell killing and low cytokine release across multiple targets, demonstrating their potential to expand the therapeutic window across solid tumor indications.
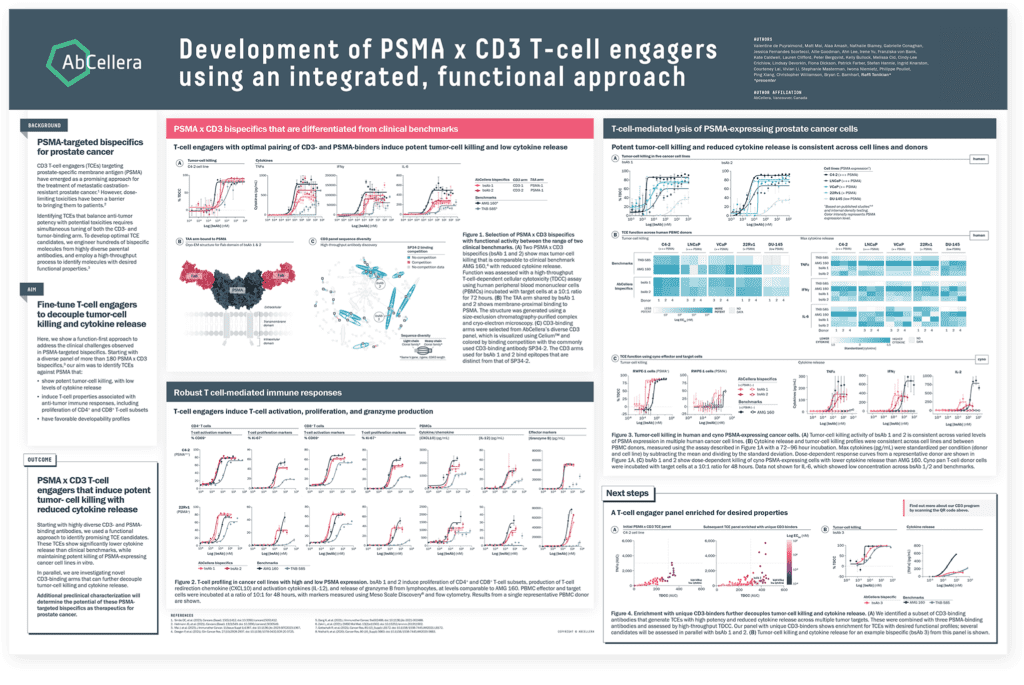
In our second poster, we presented additional data on generation of PSMA x CD3 TCEs for the treatment of castration-resistant prostate cancer. We used our function-first approach to identify TCEs that showed significantly lower cytokine release than clinical benchmarks while maintaining potent killing of PSMA-expressing cancer cell lines.
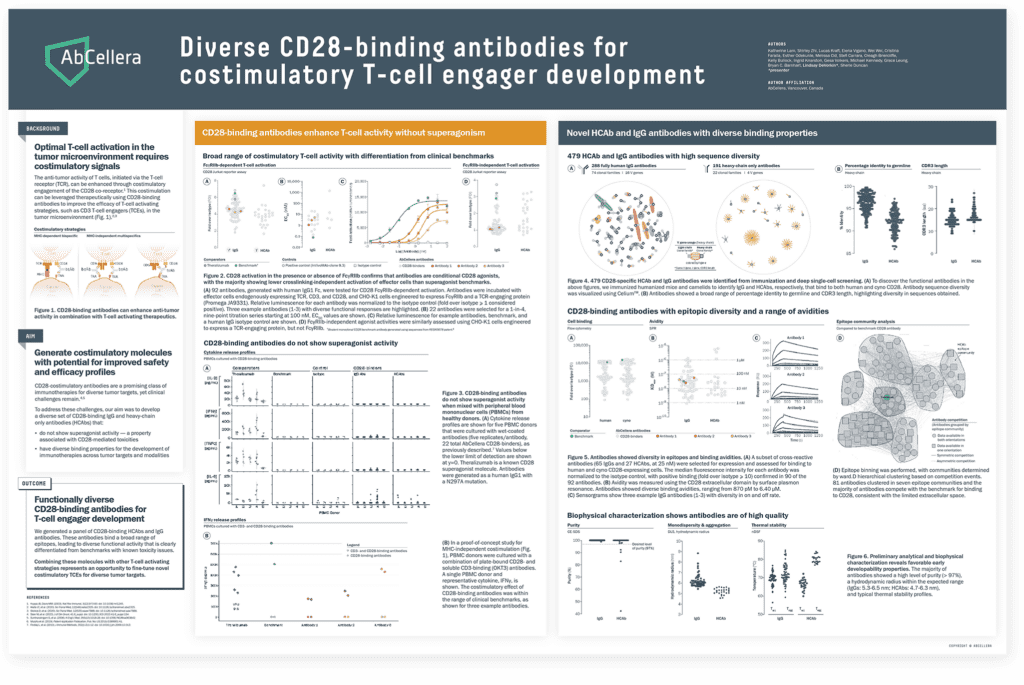
Enhancing potency
TCEs that engage the CD28 costimulatory receptor can enhance T-cell activation, proliferation, and anti-tumor activities, particularly in solid tumors. We presented data demonstrating that our IgG and heavy chain-only CD28-binding antibodies do not display superagonist activity — a property that has been associated with toxicities. Integrating costimulatory building blocks into our TCE repertoire may enable development of molecules with enhanced potency for difficult-to-treat cancers.
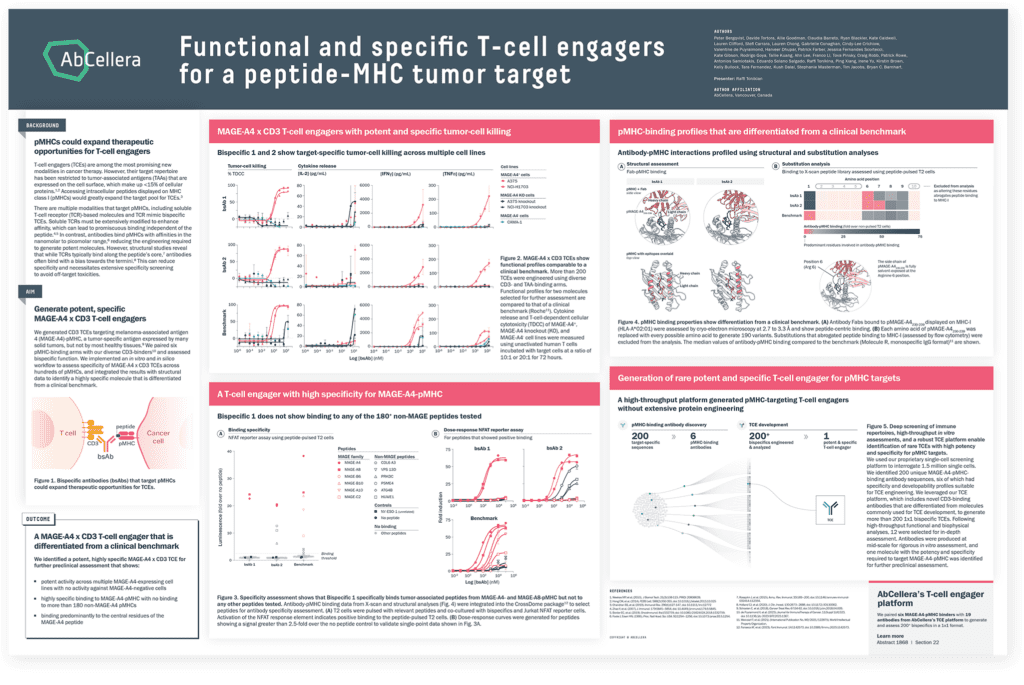
Broadening the accessible target space
The target repertoire for TCEs has been restricted to proteins expressed on the surface of cancer cells. Intracellular peptides displayed on MHC class I (pMHCs) would greatly expand the target pool for TCEs. However, development of TCEs against pMHCs has been limited due to the high degree of target specificity required. Data illustrate how our platform is unlocking this target class by generating molecules with high specificity for MAGE-A4-pMHC, which showed little to no binding to hundreds of off-target pMHCs.